So there is light that tells us what stars and planets are made of, but how do scientists get colored lines to tell us what a planet is made of?
The Scottish physicist Thomas Melvill burned different substances and looked at the resulting light through a prism. He saw spectra with different patterns than those seen when daylight passed through a prism. These spectra were not unbroken stretches of color from violet to red, but patches of color. He also saw dark gaps (missing colors) between the patches.
Later the vaporous light was passed through a narrow slit prism. The spectrum of each gas was seen as a set of bright lines of a few definite colors. The colors and locations of these bright lines were different when different substances were put in the flame.
Shining a white light through the gaseous vapor causes some energy to be absorbed by the gas (just like the ray of light travelling to Earth that I described earlier). The dark bands in the spectra show where the energies of those photons were absorbed. The remaining lines of color match up exactly with the energies of the electrons that make up the gaseous elements.
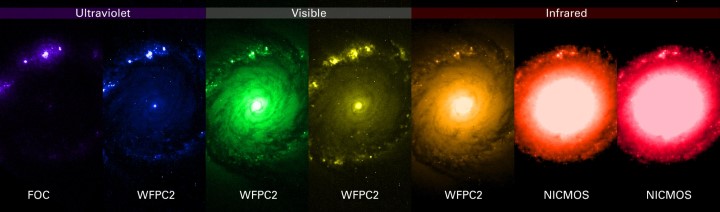
An atmosphere is made of gaseous substances. Scientists discovered that all of the elements making up an atmosphere have a unique signature (or fingerprint). By looking at the spectra of light emitted by a planet's atmosphere, scientists can tell what gases were present when that light began its journey toward the telescope.

Spectra
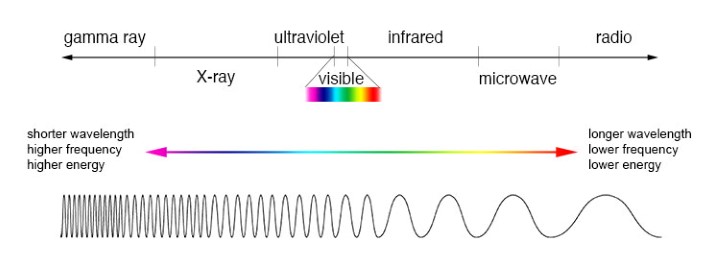
A spectrum is a visual representation of electromagnetic radiation (light) that is produced by a source, for example, a star. This image shows three types of spectra. The top band is a continuous spectrum, which shows the electromagnetic radiation emitted by a source as the result of its temperature. The middle band is an emission spectrum. This type of spectrum shows bright lines at certain wavelengths separated by dark regions where the light has been absorbed. The bottom band is an absorption spectrum, showing dark lines at the wavelengths where light has been absorbed. Because the bright and dark lines are characteristic of certain elements, astronomers can use emission and absorption spectra to determine the composition of stars and interstellar matter.
To study the atmosphere of a planet, astronomers wait until a star or the Sun shines through the atmosphere. They then look at the spectral lines, comparing them to the lines of the known elements. The same thing is done to determine what gases exist in interstellar space, in the space between galaxies, or in the atmospheres of individual stars. The Space Telescope Imaging Spectrograph (STIS) on the Hubble Space Telescope is used to analyze glowing gases of astrophysical objects through a long-slit spectrograph. A
diffraction grating is used instead of a prism. The STIS divides the light given off by these gases to reveal the fingerprints of the separate gases.
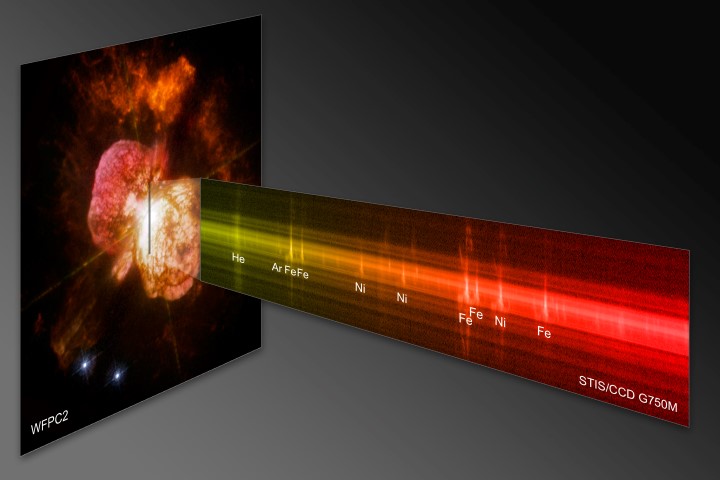
Spectral Analysis of Eta Carinae
The Space Telescope Imaging Spectrograph (STIS) on the Hubble Space Telescope analyzed the chemical information along a section of one of the giant plumes of gas from the exploding binary star system, Eta Carinae. In the resulting spectrum, iron (Fe) and nitrogen (Ni) are seen at the outer boundary of the gas cloud coming from Eta Carina A, the primary star. The faint line of argon (Ar) is evidence of an interaction between the gases from Eta Carina A and Eta Carina B, the hotter, less massive, secondary star in the system. The explosion that produced these gas clouds occurred in the mid-19th century.
Credit line here.
Questions
Why is it important to scientists interested in space exploration to know what a planet's atmosphere is made of?
 Hubble's position above Earth's atmosphere and its instruments used for making a variety of observations have given us a clear, new understanding of the Universe. This lesson presents an overview of the scientific instruments on board the Hubble Space Telescope and what what we learn from them.
Hubble's position above Earth's atmosphere and its instruments used for making a variety of observations have given us a clear, new understanding of the Universe. This lesson presents an overview of the scientific instruments on board the Hubble Space Telescope and what what we learn from them.








 Education and outreach collections from the University of Chicago
Education and outreach collections from the University of Chicago
