

|

The Scientific Article: From Galileo's New Science to the Human Genome
BY | Joseph E. Harmon | Alan G. Gross
SESSION 1: Precursors and Rivals
Books
 |
| University of Chicago Special Collections Research Center |  In Discours de la méthode, Descartes offered an explanation of the rainbow. In Discours de la méthode, Descartes offered an explanation of the rainbow. |
Long before the scientific article made its first appearance in the late seventeenth century, there were books. Without question, books and not articles communicated the new revelations about the natural world that spawned the scientific revolution. Among those making the honor roll in the sixteenth and seventeenth centuries, one would have to include Copernicus's De revolutionibus orbium coelestium, Bacon's Novum organum, Kepler's Dioptice, Galileo's Sidereus nuncius, Descartes's Discours de la méthode, and Newton's Principia. Although by the eighteenth century, the article had advanced from an insignificant competitor of the book to a serious rival in the communication of new science, books remained an important means of scientific communication well into the nineteenth century. While at times endangered, even in the twentieth century the book has not become extinct as means for reporting original research findings or making a contribution to theory. George Williams's Adaptation and Natural Selection and David Lack's Darwin's Finches, for example, are twentieth-century books relating ground-breaking evolutionary biology. Learned letters and essays
 |
| University of Chicago Special Collections Research Center |  Galileo's 1613 study of sunspots blends text and images. Galileo's 1613 study of sunspots blends text and images. |
Besides books, the other major predecessor and rival for communicating new science in the seventeenth century is the "learned letter," most famously illustrated by Galileo's letters on sunspots and the orbits of the planets. As the ideas of the scientific revolution spread in England and the Continent, the accelerated pace of scientific activity compelled natural philosophers to communicate their recent findings through personal correspondence within and between countries. Such letters were not personal messages to friends or colleagues, but an essay-like medium to expound on recent experiments and observations concerning the nature of things to others interested in such matters. To disseminate the information in these learned letters more efficiently, industrious scholars became centers for spreading the latest technical news at home and abroad. Their job was to receive letters, make copies, and pass them on to other interested scholars. After the emergence of scientific societies, the job of "trafficker in intelligence" became more formalized in that the societies themselves appointed a secretary to handle correspondence and transmit newsworthy learned letters to society members and friends. One such letter was penned in 1644 by Evangelista Torricelli, assistant to Galileo and father of hydrodynamics, and addressed to Michelangelo Ricci, a Roman cardinal and patron of science. After a typical flowery salutation of the day, Torricelli opens by briefly mentioning the whereabouts of another learned letter he had written on an entirely different topic, a mathematical proof, and sent to someone else. From that personal tidbit of information he jumps to an introductory-type discussion concerning past vacuum experiments and conjectures, which includes the vivid metaphor ,"We live immersed in the bottom of a sea of elemental air..." Torricelli wrote his learned letter at a time when Aristotle's view that a vacuum could not exist still had currency, particularly among some powerful Jesuit theologians. So he cleverly skirts that controversial issue by arguing at the beginning that some deny the existence of a vacuum, others do not, but of the latter group, no one has denied that it exists in nature without "difficulty": he thus had conducted some experiments to determine whether or not the weight of air caused the resistance of nature to a vacuum, in case one indeed existed.
 |
| University of Chicago Special Collections Research Center |  A flea from Hooke's Micrographia. A flea from Hooke's Micrographia. |
The letter never really disappeared as a medium of scientific communication: personal letters between scientists remain a vital element in any historical reconstruction of their science. And as indicated by the letters in Nature, Science, Physical Review Letters, and other contemporary journals, the published letter is still a significant medium of scientific communication. Moreover, the link between the learned letter and the article is direct: many of the articles in the very first journals are learned letters lightly revised for publication by an editor. This was the case with Newton's famous first article on optics, published in the 1672 Philosophical Transactions. Also important during the seventeenth and eighteenth centuries were books containing collected letters or short articles on technical matters by a single author, as exemplified by Leeuwenhoek's published letters to the Royal Society of London and Hooke's splendidly illustrated Micrographia. |
|
SESSION 2: Origin and Early Years
First English journal and the Royal Society
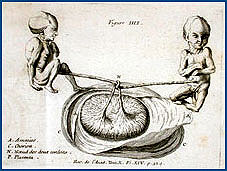 |
| Univ. of Chicago Special Collections Research Center |  Representation of two fetuses establishing a cause for their demise--a knot in the umbilical cord. Representation of two fetuses establishing a cause for their demise--a knot in the umbilical cord. |
It was in March 1665 that Henry Oldenburg, the secretary of the Royal Society of London, launched the first English scientific journal, Philosophical Transactions. His transactions reported on technical news from abroad, as well as articles presented at the Royal Society and experiments performed there--or in the words on the title page, "Giving some accompt (sic) of the present undertakings, studies, and labours of the ingenious in many considerable parts of the world." The initial transactions, issued monthly, were about 20 pages long and printed in runs of 1,200 copies. The first issue had contributions from, among others, the eminent scientist and Royal Society Fellow Robert Hooke, an astronomer, an "inquisitive Physician," and an "understanding and hardy Seaman." This modest yet historic first issue concluded with an obituary for the French mathematician Pierre de Fermat. The author index under Oldenburg's stewardship (1665-1677) reads like a who's who of seventeenth-century scientists in England and Europe: Newton, Boyle, Hooke, Wren, Leeuwenhoek, Huygens, Hevelius, Leibnitz, Cassini, and Halley. Oldenburg's journal was unofficially associated with the Royal Society, and its members and their friends in Europe were the main contributors. According to Bishop Sprat's History of the Royal Society (1667), society members sought to reject "amplifications, digressions, and swellings of style...bringing all things as near the Mathematical plainness, as they can: and preferring the language of Artizans, Countrymen, and Merchants, before that, of Wits and Scholars." Despite Sprat's distrust regarding "this trick of Metaphor, this volubility of Tongue," some early science writers did manage to make effective use of it. Take the following metaphorically rich botanical description by Martin Lister, a physician with a keen interest in entomology and botany, as well as a frequent early contributor to Philosophical Transactions. In the middle of July, I drew and gathered of the Milk of Lactuca syl. Costa spinosa, C.B. and of all our English Plants, that I have met with, this most freely and plentifully affords it. It springs out of the Wound thick as Cream and Ropes, and is White, and yet the Milk which came out of the Wounds, made towards the top of the Plant, was plainly streaked or mixt with a purple Juice, as though one had dashed or sprinkled Cream with a few drops of Claret. And indeed, the Skin of the Plant thereabouts was purplish also, perhaps with Veins. Again, in the Shell I drew it, it turned still yellower and thicker, and by and by curdled, that is, the white and thick caseous part did separate from a thin purple Whey. So the Blood also of Animals, whilst warm remains liquid and alike, but so soon as cold, it cakes and has a Serum or Whey separated from it; the Cake is made of glutinous Fibers, and therefore if the hot or new drawn Blood be well stirred or beaten, it will not break.
While Philosophical Transactions has seen writing styles come and go, it has maintained its emphasis on "Mathematical plainness" and is still going strong today. First French journals and Académie Royale
The other major learned journal from the seventeenth century, Journal des sçavans, was started in January 1665 by Denis de Sallo. Before starting this weekly journal, Sallo had copyists transcribe especially interesting passages from books and letters so that he could quickly access information on a broad range of topics. Recognizing that other scholars might also profit from this diverse stockpiling of information, this polymath decided to publish weekly book reviews and news in science, as well as in law and theology. Because the Journal des sçavans began primarily as a review of technical and other scholarly works, some scholars bestow the honor of "first" scientific journal on Philosophical Transactions, even though Sallo founded his journal two months earlier. In Sallo's own words, his journal was instituted "for the relief of those either too indolent or too occupied to read whole books. It is a means of satisfying curiosity and of becoming learned with little trouble." Under the editorship of Sallo and his successors, the journal reported some of the most important discoveries in the world of seventeenth-century European science, including Huygens's undulating theory of light and Roemer's measurement of its velocity. Before his fellow members of the French Académie Royale on September 1676, the Danish-born Ole Roemer predicted that the eclipse of one of the moons of Jupiter would occur on November 9, 1676, at 5 hours, 35 minutes, and 45 seconds; that is to say, exactly ten minutes after the time computed on the basis of an instantaneous speed of light. Roemer used the regular pattern in his observed discrepancies in the time for the eclipses of a moon of Jupiter (Io) over time to argue that they resulted because light has a definite speed. As this moon and the earth grew farther apart, then closer together, the time for light to reach the earth changed accordingly. His prediction was confirmed by the Académie's Royal Observatory in Paris. According to Roemer's calculations, published in the 1676 Journal des sçavans (and translated in the next year's Philosophical Transactions), the speed of light was such that it required roughly 22 minutes to travel the diameter of the earth's annual orbit. That equals approximately 215,000 kilometers per second, reasonably close to the actual value of 299,792. 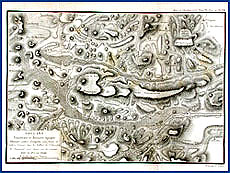 |
| Univ. of Chicago Special Collections Research Center |  Map illustrating the past history of volcanic activity in France. Map illustrating the past history of volcanic activity in France. |
Up to the very early eighteenth century, the Journal des sçavans was the periodical that published the research activities of the Académie Royale des Sciences in Paris. That changed in 1702, when the Académie Royale started the regular publication of its researches in its Histoire et Mémoires. The Histoire contained summaries of the Mémoires, which were selected full-length articles arranged in roughly chronological order and written by academy members. The Académie's Mémoires is widely viewed as the premier scientific journal in the eighteenth century. First journals from other countries
The first scientific societies--part social club, part research institute, part publishing house--constitute the major institutional factor in shaping the scientific article at and just after its origin. These neophyte organizations had within their ranks most of the authors, readers, and editors of seventeenth-eighteenth century science in England and the Continent. They also created the social networks needed to establish what constituted good science and acceptable scientific prose. The elegant Saggi di naturali esperienzi (Essays of natural experiments), written in Italian and published in 1667 by the short-lived Accademia del Cimento in Florence, is the first important record devoted exclusively to a scientific society's activities. This masterpiece of early bookmaking describes instruments and procedures and gives results for an eclectic collection of experiments performed by society members during 1657-1662. The Saggi was the only publication by this early scientific society and became the laboratory manual for physics experiments in the 17th and 18th centuries. The two early journals from the German-speaking lands--Acta eruditorum (founded in 1682) and Miscellanea curiosa medico-physica (1670)--adopted Latin to reach the widest possible international audience. Among other important discoveries, the Acta published most of the articles in which Leibnitz announced and articulated his invention of the calculus. The Miscellanea, a product of the Collegium Naturae Curiosorum, was indeed a potpourri of curiosities. The 160 observations in the first year's volume include a remedy for an atrophy of the eye, a report on a serpent petrified in a stag's stomach, an account of a horny substance growing out of a man's rib cage, cures for deafness and headache, a way of "dwarfing men," and tales of men and women willing to eat strange things. By the mid-eighteenth century, most major cities and many provincial ones had their own scientific societies, each with its own acta, commentaries, journal, proceedings, transactions, miscellanea, or memoirs. Early scientific communication in America
Scientific societies and their publications took root in America even before the Declaration of Independence. The first American scientific society was the American Philosophical Society, started by Benjamin Franklin, among others, in Philadelphia in 1743 to pursue "all philosophical Experiments that let Light into the Nature of Things." After petering out for lack of interest after less than four years, the society was revived permanently in 1768. The first volume of its Transactions (1771) contains many articles reporting observations and measurements of the transit of Venus across the sun in 1769 and helped establish an international reputation not only for the American Philosophical Society, but American science in general. Thomas Jefferson served as the society's third president (1797-1815) and published a paleontological article on a sloth fossil in the fourth volume of its Transactions. The early American journals and scientific books are filled with new flora and fauna found throughout North America, and brought to life in both verbal descriptions and artistic engravings. John James Audubon's work on birds is the most widely known example. But many others, both native and foreign visitors, also contributed to the enormous enterprise of systematically documenting the splendors of the various species inhabiting the new world.As in many early journals, the American publications mixed scientific observation with fabulous bits of hearsay. In the same volume where Jefferson's article, "A Memoir on the Discovery of Certain Bones of a Quadruped of the Clawed Kind in the Western Parts of Virginia," appears is "A Letter from Mr. John Heckewelder to Benjamin Smith Barton, M.D. containing an Account of an Animal called the Big Naked Bear." Other early American journals include Memoirs of the American Academy of Arts and Sciences (1785), Journal of the Academy of Natural Sciences (1817), and the American Journal of Science and Arts (1818). The fact-gathering enterprise of early science
The scientific community of the seventeenth and eighteenth centuries had a large amateur constituency; these science enthusiasts were not especially concerned with concocting mathematical or mechanical explanations for their observational or experimental results. Many articles are simply undigested observations such as the sighting of comets or eclipses, descriptions of microscopic organisms and exotic flora and fauna, and accounts of medical oddities and other "strange facts." Suspicious of theorizing and speculation beyond the bare facts, many natural philosophers of that age saw themselves as participating in Francis Bacon's great unrealized dream of a museum containing a specimen of every scientific fact. 
University of Chicago Special Collections Research Center | Illustrations from Claude Perrault's "Anatomical description of three chameleons," published in 1700 by the French Académie Royale. The Académie began publishing Mémoires pour servir à l'histoire naturelle des animaux, a volume dedicated to zoological research, in 1671. |
 |
| National Agricultural Library |  Hibiscus from Curtis's Botanical Magazine. Hibiscus from Curtis's Botanical Magazine. |
One concrete manifestation of Bacon's dream was the fact-driven journal Curtis's Botanical Magazine (1787). Each issue of the magazine contains a series of vibrant watercolor depictions of plants rendered in lovingly exact detail. Until 1948, the magazine manufactured these artistic gems by mass-producing a line drawing by a professional artist or the author, then having groups of women and children hand color each illustration. Accompanying each illustration is a page or two of verbal description, including such technical details as the plant's habitat, color, scent, size, flower placement, and method of pollination.Another manifestation of Bacon's dream was the monumental French encyclopedia of the eighteenth century, Encyclopédie: ou Dictionnaire raisonné des sciences, des arts et des métiers. The closeness of the full title to journal names of that time--such as Observations et mémoires sur la physique, sur l'histoire naturelle et sur les arts--attests to their kinship. And like Curtis's Botanical Magazine, the entries in the encyclopedia approached the subject matter in a systematic way regarding writing style, format, and content and also relied heavily upon visual representations, in this case nearly 3,000 copper-plate etchings. |
SESSION 3: Specialization and Growing Maturity
Origin of the specialized literature
In the beginning was the General Scientific Journal. And the General Scientific Journal begat the Specialty Journal, and the Specialty Journal begat the Single-Subject Journal, whether according to class of compounds, specific disease, or methodology. And the Single-Subject Journal begat the Interdisciplinary Journal to link up the specialties at an earlier evolutionary date. And the scientific community saw the journals were good, and they were fruitful and multiplied.
Eliot M. Berry, in 1981
New England Journal of Medicine In the later decades of the eighteenth century, scientists along with their societies and publications became more specialized as a means of coping with the flood of technical knowledge, particularly in the fields of physics and chemistry. The age of the generalist and inspired amateur of science was in decline.  |
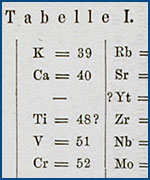
| University of Chicago Special Collections Research Center |  Before the periodic table of elements, scientists used arcane symbols to organize chemical "substances." Before the periodic table of elements, scientists used arcane symbols to organize chemical "substances." |
One of the first general scientific journals aimed at serious researchers was the Observations et mémoires sur la physique, sur l'histoire naturelle et sur les arts, founded in 1773 by François Rozier. As Rozier eloquently, if brusquely, put it in the preface to the first volume: "We will not offer to idle amateurs purely agreeable works or the sweet illusion of believing themselves to be initiated into science of which they know nothing...We offer this collection to the truly knowledgable." He further asserted that the journal itself would "reject everything that is nothing more than undigested compilation and that is wanting in new and useful views." At the founding of the British Association for the Advancement of Science in 1831, William Whewell suggested that membership be restricted to those "who have published written papers in the memoirs of any learned society." He wanted to exclude as members those who were not, as one critic of the Royal Society put it, "labourers in the vineyard" of science. This general desire for higher professional standards in science led to an influx of individual articles primarily aimed at subject-matter experts. It also spawned the first great specialty journals in the natural and physical sciences from Germany, France, and England.
Types of scientific article
Over the centuries, five types of scientific article have emerged: theoretical, experimental, observational, methodological, and review. These types are distinct, each having a different purpose, though closely intertwined. Theoretical articles focus on explaining natural events, often suggesting experiments or observations that might confirm the explanations. They offer the new conceptual variations that drive the continued evolution of science. Experimental articles recount the manipulations of natural objects, usually in artificial settings such as research laboratories. They provide the empirical information essential for the continued conceptual evolution of science. Observational articles describe natural objects, usually outside the laboratory. They involve such activities as describing a new hummingbird or fossil or measuring the spectrum of a new star; they do not primarily involve manipulating natural objects under controlled conditions. Observational articles complement the experimental; they exist because part of the task of science will always involve describing the natural world outside the laboratory. In addition, as cosmology and paleontology demonstrate, some phenomena that interest science will always be closed to direct experimentation. Methodological articles do not usually make new claims about the natural world but present new means for facilitating and creating experiments and improving observations. They are about the tools used to create new science. Review articles describe and evaluate the recent literature in a field; they usually contain no major claims not presented in previous articles. While their purpose is to interpret past science, not invent new science, they serve an indispensable function--winnowing the fit from the unfit among the other four types. They constitute a second tier of peer review, one far more selective than the first. Invention of the modern graph
Scientific articles with no graphs? For the present-day reader that's hard to imagine. Yet, the modern graph was not "invented" until the late eighteenth century, a hundred years after the scientific journal made its debut. At that time, it was used extensively by the German physicist Johann Heinrich Lambert and the English economist William Playfair.  |
| University of Chicago Special Collections Research Center |  William Playfair was the first to use color graphs to show economic data. William Playfair was the first to use color graphs to show economic data. |
In this visual form, the author typically plots some independent variable, such as time, on the abscissa (horizontal axis) and some dependent variable, such as a physical or chemical property that changes over time, on the ordinate (vertical axis). This arrangement is ideally suited for communicating multiple data points at a glance, visually representing cause and effect, uncovering trends in a large mass of data, and making comparisons among multiple data sets. As astronomer J. F. W. Herschel noted in an 1833 article, "Such charts [blank ones with pre-drawn axes]...are so very useful in a great variety of purposes, that every person engaged in...physico-mathematical inquiries of any description, will find his account in keeping a stock of them always at hand." The steadily increasing use of graphs during the nineteenth and twentieth centuries has greatly contributed to the scientific article moving away from the descriptive and qualitative, toward the mathematical and quantitative. The graph now has to be considered the premier form for scientific visual representation, supplanting geometric diagrams and realistic drawings of natural objects and research equipment.Invention of the periodic table of elements
 |
| University of Chicago Special Collections Research Center |  Dimitri Mendeleev's periodic table became one of the foundations of modern chemistry. Dimitri Mendeleev's periodic table became one of the foundations of modern chemistry. |
Similar to graphs during the nineteenth and twentieth centuries, tables also became a more common ingredient of the scientific article as a means of coping with its ever expanding reliance on the quantitative. One of the most remarkable and wonderful of all tables in science has to be Dimitri Mendeleev's periodic table, the backbone of modern chemistry. In three 1869 articles and the book Osnovy khimii (The principles of chemistry), Mendeleev published the first versions of his table, which organized the 65 known chemical elements into rows and columns guided by the increasing order of their atomic weights and chemical properties. According to Mendeleev, "The definition of [atomic] mass gave a means of analyzing and grasping chemical transformations of substances, and for arriving at the atom, while the mass of the atom was shown by the periodic law to influence all its chief chemical properties" (trans. from Russian by George Kamensky). More important, in an 1871 article he forecast that gaps in the table would be filled by the discovery of new elements with predictable properties. Mendeleev's prediction was confirmed with the discovery of gallium in 1875, scandium in 1879, and germanium in 1885. In 1913, Henry Moseley reported that the number of protons in a given element (atomic number) worked better than atomic mass as a basis for arranging the periodic table. During the twentieth century, scientists have discovered many new elements predicted by the periodic law and invented many new arrangements of the periodic table, including spiral and triangular versions, as well as ones designed for the World Wide Web. Since the mid-1990s, scientists have welcomed into the periodic fold six more elements, the heaviest being elements 116 and 118 (brand new as of June 1999). Women and minorities in science
In the seventeenth century, the Royal Society of London was open to just about any gentleman who could pay its dues: not only full-time researchers, like Newton and Boyle, but also those with an above-average curiosity about things scientific and mathematical, like John Dryden, poet laureate of England. A learned woman, Margaret Cavendish, the duchess of Newcastle, even attended a meeting in 1667. Sadly, this society's first woman "fellow" was not elected until 1945. While women are largely absent from learned societies and the pages of scientific journals until the early twentieth century, a few did work as technical assistants (for the most part, unpaid and unacknowledged), while still others played an important role in communicating scientific knowledge through books published in the seventeenth through nineteenth centuries. In the late eighteenth century, Marie-Anne Lavoisier prepared technical illustrations for her husband's chemical publications, lent a hand in his laboratory, and translated a scientific book on phlogiston. At roughly the same time, Caroline Herschel published a Catalogue of Stars under the auspices of the Royal Society of London. In fact, Herschel sighted eight new comets in her long life, three of which she reported in very brief Philosophical Transactions articles.  |
| University of Chicago Special Collections Research Center |  A plate from Maria Sibylla Merian's lavishly illustrated Metamorphosis insectorum Surinamensium A plate from Maria Sibylla Merian's lavishly illustrated Metamorphosis insectorum Surinamensium |
One could make a convincing case that Marie Curie is the first woman to publish scientific articles of major significance, thrusting her into the front ranks of important scientists worldwide. In the year 1898, aged 30, she along with her husband published two important articles, each announcing the discovery of a different radioactive element detected in the uranium ore called pitchblende. The first article reported on polonium, named after Curie's native country; and the second, radium, named from the Latin word for "ray."
Another woman physicist of importance is Lise Meitner. In 1938 Otto Hahn and Fritz Strassmann, working in the physics department at the Kaiser Wilhelm Institute, split the atom of uranium. Shortly thereafter, Meitner and her nephew Otto Frisch provided an explanation on why Hahn and Strassmann's bombarding uranium (atomic number of 92) with neutrons formed isotopes of barium (atomic number of 56) when an element with atomic number close to uranium was expected. To solve this problem, Meitner and Frisch applied the liquid-drop model of the nucleus first proposed by Niels Bohr. In this model, they pictured uranium as a liquid drop, which becomes unstable when hit by a neutron, elongates into a dumbbell shape, then divides into two smaller drops. This process leads to two smaller nuclei (barium and krypton), releasing considerable energy (200 million electron volts) in conformance with Einstein's famous equation E = mc². They called this process "fission," borrowing a term from biology. In the late nineteenth century, the doors of universities, scientific societies, and research laboratories gradually opened to women. As a consequence, in the twentieth century, one finds many women authors making major contributions to the scientific literature. Other groups have also been shunned from the practice of science, just as in other professions. Ernest Everett Just, one of the first prominent African-American biologists, left the United States in the early 1930s to work in Europe because he felt unappreciated and discriminated against in his native land. During his long but tumultuous career, he published about 70 scientific articles and two technical books. |
SESSION 4: Select Communications from Ingenious Scientists
Newton and optics
The eightieth issue of Philosophical Transactions features an article by an up-and-coming young scientist, Isaac Newton--his first scientific publication and the first major scientific article ever. Henry Oldenburg, the Transactions editor, obviously recognized the importance of this 12-page article by the relatively obscure Cambridge University professor, because it is the sole research article in the eightieth issue and was printed only about two weeks after its reception, on February 6, 1672, in handwritten manuscript form. Here is the extensive byline preceding the actual article: A Letter of Mr. Isaac Newton, Mathematick Professor in the University of Cambridge; containing his New Theory about Light and Colors: Where Light is declared to be not Similar or Homogeneal, but consisting of difform rays, some of which are more refrangible than others: And Colors are affirm'd to be not Qualifications of Light, deriv'd from Refractions of natural Bodies, (as {A145}tis generally believed;) but Original and Connate properties, which in divers rays are diverse: Where several Observations and Experiments are alledged to prove the said Theory.
Newton's main contention was that white light, far from being simple, as previously believed, was a compound of all the colors of the spectrum, a compound that could be decomposed by passing white light through a prism and recomposed through reversing that passage in a second prism. The claim had two basic components: first, Newton's experimental results with prisms and, second, his mechanical explanation for them--namely, that light appears to be a material body composed of particles, and rays of differently colored light refract at different angles. The initial reception of Newton's article by the Royal Society fellows was largely positive: his article had an early impact on thinking about light and color in England and on the Continent, and it was taken seriously by the two most prominent authorities on the physics of light, Robert Hooke and Christiaan Huygens. Nonetheless, all did not go smoothly. Several virtuosi challenged his experimental results, and Hooke and Huygens offered plausible alternative explanations to Newton's, that light rays behaved as pulses or waves. This controversy marks the first important public difference of opinion within the emerging community of science. Newton's final response to his critics came in the form of a book, Opticks, first published in 1704. Darwin and evolutionary theory
 |
| University of Chicago Special Collections Research Center |  A journal containing articles by Charles Darwin and Alfred Wallace. A journal containing articles by Charles Darwin and Alfred Wallace. |
The Darwinian revolution begins as a story of a young man whose father is properly anxious about his son's future, yet he agrees, with considerable misgivings, to allow his son to sail with the Beagle, a ship whose mission in circumnavigating the globe was to map the coasts of South America and to collect specimens of flora and fauna. For Darwin, the journey, which lasted five years (1831{A150}1836), was a voyage of intellectual discovery. But it was a voyage only begun on the Beagle; it was nearly a quarter of a century before Darwin published on evolution, and even then, his motive for publication was not his growing sense of certainty, but the receipt of an article by Alfred Russel Wallace, who had discerned in a flash of insight the central tenet of evolution--that its driving force was natural selection operating on variation of species in an environment of limited resources. The Darwinian revolution was successful only in retrospect. Even with the help of allies, most famously, Thomas Henry Huxley, Darwin's theory faced considerable opposition, not only on religious, but also on scientific grounds. For example, Darwin's theory presupposed the heritability of better adapted traits, but his original theory did not satisfactorily address this crucial issue. Ironically, six years after the Darwin{A150}Wallace articles and the Origin were published, so was a solution to Darwin's problem. Gregor Mendel's first article on heritability in the common pea was published in 1866. But it appeared in an obscure periodical and was written by a scientist equally obscure. It had no immediate influence and had to be "rediscovered" early in the following century. Einstein and relativity theory
 |
| University of Chicago Special Collections Research Center |  First edition of a lecture on relativity given by Albert Einstein in 1920. First edition of a lecture on relativity given by Albert Einstein in 1920. |
The year 1905 has to be considered an annus mirabilis in the long history of the scientific article. For that is the year Albert Einstein--a technical expert in the Swiss patent office freelancing on theoretical physics problems--published four ground-breaking articles in Annalen der Physik. They included not only his first two articles on relativity theory, but also one offering a mathematical explanation for Brownian motion, and another proposing a mechanical explanation for the photoelectric effect. Over the next decade Einstein continued to build his "principle of relativity" in a remarkable series of articles altering forever our notions of time, space, motion, gravity, light, mass, and energy. The 1905 article entitled "Ist die Trägheit eines Körpers von seinem energieinhalt Abhängig? " (Does the Inertia of a Body depend on its energy Content?) is typical of Einstein's relativity articles: a Euclidean deduction from some basic postulates leads to a startling prediction--the equivalence of mass and energy, E = mc². This revelation is a mathematical expression directly in accord with Einstein's philosophy of science. According to this philosophy, physics is the search for laws that describe relationships among fundamental entities that completely determine physical laws. "I still believe in the possibility of a model of reality," wrote Einstein in 1954, "that is to say, of a theory which represents things themselves." Fermi and nuclear research
More than a half century ago Enrico Fermi stood in Squash Courts under the stands at Stagg Field, a football arena abandoned at the end of the 1939 season, the last inglorious year of Division I football at the University of Chicago. It was the morning of December 2, 1942, and the first atomic pile was about to go critical. Here is how Fermi described the events of that day: ...the indications were that the critical dimensions had been slightly exceeded and that the system did not chain react only because of the [neutron] absorption of the cadmium strips. During the morning all the cadmium strips but one were carefully removed; then this last strip was gradually extracted, close watch being kept on the intensity. From the measurements it was expected that the system would become critical by removing a length of about eight feet of this last strip. Actually when about seven feet were removed the intensity rose to a very high value but still stabilized after a few minutes at a finite level. It was with some trepidation that the order was given to remove one more foot and a half of the strip. This operation would bring us over the top. When the foot and a half was pulled out, the intensity started rising slowly, but at an increasing rate, and kept on increasing until it was evident that it would actually diverge. Then the cadmium strips were again inserted into the structure and the intensity dropped to an insignificant level. [From Proceedings of the American Philosophical Society, 1946] Soon after, Arthur Holly Compton, the project leader at Chicago, made a call to James Bryant Conant in Washington. Since the call was made on an insecure line, the conversation was in an impromptu code. "Jim," Compton said, "you'll be interested to know that the Italian navigator has just landed in the new world." Conant wondered "Were the natives friendly?" "Everyone landed safe and happy" was Compton's reply. Throughout his illustrious career, Fermi published extensively on his nuclear research, winning the 1938 Nobel Prize for his discovery of "new radioactive elements produced by neutron irradiation, and...nuclear reactions brought about by slow neutrons." Understandably, his journal publications came to an abrupt halt in 1941 and then resumed after the war. |
SESSION 5: Miscellanea Curiosa
Failure and science
The literature of science is as much about wrong turns, aborted lines of inquiry, failure to thrive, and outright failure as success. Even the greatest of scientists fails now and again, just like the rest of us. Indeed, unless one is selective to the point of unrepresentativeness, it must be admitted that much of past science is a collection of dead ends and well-intentioned mistakes, eventually to be discarded. These failures can take many general forms. They can be discoveries that after a short time are proved erroneous, as was the case with N-rays, cold fusion, and polywater. They can also be concepts with a long, successful track record that are found to be invalid or somehow theoretically inadequate, such as phlogiston, light particles, and the universal ether. They can also be ambitious research programs that appear, from the vantage of hindsight, to have been futile because the then-current body of knowledge or research techniques were too primitive to permit a major breakthrough, as in Einstein's failed attempts at constructing a unified field theory. Some research projects fail to survive because of a loss of financial backing, as was the case with the effort to build a colossal superconducting supercollider in Texas. And of course experiments, observations, or theories can be flawed in some small or large way: to error is human and part of the discovery process.Smaller errors, once detected, may be announced in the "Erratum" section of a scientific journal. These entries are usually short and straightforward, but one example shows an author mercilessly disparaging his own published work. In an item in the Journal of Chemical Physics (vol. 47, 1967) titled "Erratum: Charge Exchange between Homonuclear Diatomic Molecules and Protons," R.G Breene, Jr. summed up his views on an article he had published in the Journal the previous year: "The material contained in this article is nonsense and should be discarded in toto. As will be shown elsewhere, one of the most serious defects is the poor transformation given by Eq. (9). It is to be emphasized that the printer set the manuscript precisely as the author had concocted it." Argument and controversy
To do science is to assert that something is true of the natural world and to be prepared to defend that claim before a community of peers. In short, to do science is to make arguments and to argue. And nowhere is the argumentative nature of science more apparent than in the give-and-take of a controversy. It is during such episodes that arguments for or against a new knowledge claim are most severely put to the test. One can also find emotional outbursts at odds with the image of the scientist as dispassionate seeker of the truth.
On any short list of key scientific controversies in the twentieth century one would have to include the continental drift theory--that at one time the earth's surface housed a single supercontinent, which broke apart some 225 million years ago. This bold new theory made its first substantive appearance in a pair of 1912 articles by the German meteorologist Alfred Wegener. For decades, Wegener's theory remained "a beautiful dream" because it lacked any plausible mechanism capable of propelling such large land masses over thousands of miles. It was dismissed within the scientific community as a "German theory," "heretical," and "bizarre." That all changed in the 1960s with the seafloor-spreading theory and plate tectonics, which offered a plausible mechanism for continental drift. At the same time, a small band of holdouts, led by a distinguished Soviet geophysicist, V. V. Beloussov, and a father-son team from Oklahoma, the Meyerhoffs, fought tenaciously against the rising tide of drift theory and proposed their own variant theories, ultimately to no avail. Literary curiosities: For better or verse?
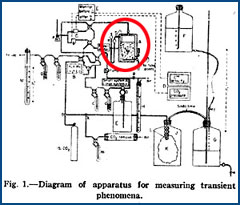 |
| American Chemical Society |  Hidden within a diagram of an apparatus from a 1955 article is a tiny fisherman. Hidden within a diagram of an apparatus from a 1955 article is a tiny fisherman. |
The poet and novelist entertain their audiences by drawing upon a great bag of literary devices, such as metaphor and simile, onomatopoeia, irony and satire, and pun. In contrast, the scientist-writer is expected to transmit information accurately and unambiguously as possible to their intended readers, not dazzle them with literary virtuosity. Anyone who reads the scientific literature is all too aware that the prose style tends to be maddeningly drab and predictable. Despite these restraints on individual creativity, scientists have managed to slip into their prose occasional literary sleights of hand. Most famously, the great German chemists of the nineteenth century, Justus Liebig and Friedrich Wöhler, published an anonymous article in the 1839 Annalen der Pharmacie reporting fictitious experiments meant to ridicule recently reported microscopic observations indicating that yeast is a living organism capable of converting sugar into alcohol. A sentence gives the flavor of this strange parody: "To summarize, sugar is sucked into the stomach of this little animal, a stream of alcoholic fluid gushes out continuously from the anus and short bursts of carbon dioxide are squirted out an enormous genital organ" (translated by Irving M. Klotz). Other scientists have constructed elaborate puns and metaphors, imported literary quotations and famous paintings into their text, hidden strange images in technical illustrations, written articles in verse, and even provided musical notes to sing along with. What we are privy to in such articles is not in any way business as usual, but science on holiday. Or in the case of Lord Kelvin, scientists on holiday. One of Lord Kelvin's many interests was the physical properties of light and its perception by humans. One day in August 1899, perhaps on a vacation in the Alps, he rose before dawn to make some observations about the first light of dawn. Fortunately for us he wrote them down in a Nature article that is a combination of a prose poem and scientific report. Hotel du Mont-Revard, August 27, 1899 Looking out at 4 o'clock this morning from a balcony of this hotel, 1545 metres above sea-level, and about 68 kilometres W. 18° S. from Mont Blanc, I had a magnificent view of Alpine ranges of Switzerland, Savoy, and Dauphiné; perfectly clear and sharp on the morning twilight sky. This promised me an opportunity for which I had been waiting five or six years; to see the earliest instantaneous light of sunrise through very clear air, and find whether it was perceptibly blue. I therefore resolved to watch an hour till sunrise, and was amply rewarded by all the splendours I saw. Having only vague knowledge of the orientation of the hotel, I could not at first judge whereabouts the sun would rise; but in the course of half an hour rosy tints on each side of the place of strongest twilight showed me that it would be visible from the balcony; and I was helped to this conclusion by Haidinger's brushes when the illumination of the air at greater altitudes by a brilliant half-moon nearly overhead, was overpowered by sunlight streaming upwards from beyond the mountains. A little later, beams of sunlight and shadows of distant mountains converged clearly to a point deep under the very summit of Mont Blanc. In the course of five or ten minutes I was able to watch the point of convergence travelling obliquely upwards till in an instant I saw a blue light against the sky on the southern profile of Mont Blanc; which, in less than one-twentieth of a second became dazzlingly white, like a brilliant electric arc-light. I had no dark glass at hand, so I could not any longer watch the rising sun. You might wonder about the term "Haidinger's brushes." According to a lecture on optics theory Kelvin delivered to the Academy of Music, Philadelphia, in 1884: "The discoverer is well known in Philadelphia as a mineralogist, and the phenomenon I speak of goes by his name. Look at the sky in a direction of ninety degrees from the sun, and you will see a yellow and blue cross, with the yellow toward the sun, and from the sun, spreading out like two foxes' tails with blue between, and then two red brushes in the space at right angles to the blue. If you do not see it, it is because your eyes are not sensitive enough, but a little training will give them the needed sensitiveness." These brush-like patterns also appear when a viewer looks at a bright surface through a polarizer.
|
SESSION 6: Conclusion, and a Look at What's Next
Writing style
 |
 Ways of organizing and presenting scientific information continue to evolve, as can be seen in the Royal Chemical Society's Visual Elements Periodic Table. Ways of organizing and presenting scientific information continue to evolve, as can be seen in the Royal Chemical Society's Visual Elements Periodic Table. |
The first scientific articles are typically brief reports (seldom more than 10 pages, sometimes a single paragraph) describing an individual's encounter with nature through observation and experiment. Particularly in England, they tend to be a run of Baconian "facts" woven into a narrative loosely connected, if at all, with an explanatory theory. In general, they seek to establish credibility by means of reliable testimony more than painstaking technical details, and by qualitative experience more than quantitative experiment and observation in support of theory. The early articles also largely rely on everyday language and are written in a style conforming to the compositional norms of expository prose generally, not norms specific to science. For the most part, the targeted audience is a loosely knit community of amateurs and professionals united by a curiosity about how nature works. The modern scientific article, in contrast, has evolved a specialized language and prose style adapted for efficient communication to other professionals engaged in similar research. The impersonal style of these articles is designed to focus the reader's mind on the things of the laboratory and the natural world, rather than to draw attention to the text itself or its author. Presentational features
The presentational features of the scientific article have evolved in two primary ways. First is development of a more uniform and regimented arrangement of the article's overall content. This arrangement represents a tribute to the efficacy of the scientific method as a means of exploring nature. To that end, the modern scientific article is typically divided into an introduction, which places readers in the scientific context in which its authors are working, a section on methods and materials that outlines the procedures used, a section on results that displays the data generated and the intellectual context of their acquisition, a discussion section that interprets the data and addresses future research that would extend the present insights, and a conclusion that reiterates the central argument in a single paragraph or two and brings the main body to a close.
The scientific article has also evolved a master finding and organizing system. This system compartmentalizes the essential features in articles through the use of summaries and abstracts, headings and subheadings, tables and figures integrated into the text, citations that supply context for statements at any point in the text, and so forth. This system permits scientists to read articles opportunistically rather than sequentially, scanning the various sections in search of useful bits of method, theory, and fact.
The latter half of the twentieth century has seen a great flowering of scientific style guides and manuals, which have codified presentational as well as stylistic features in the scientific article and contributed to its growing uniformity across national boundaries and disciplines. Visual displays
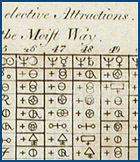 |
| University of Chicago Special Collections Research Center |  Torbern Bergman's table was an early attempt to represent chemical equations. Torbern Bergman's table was an early attempt to represent chemical equations. |
At the origin of the scientific article in 1665, several types of visual representation had already reached maturity: tables of data had long been a staple of the astronomical literature; three-dimensional drawings of anatomical features had attained a high level of technical detail and artistry, as shown by the graphics in the work of Vesalius and Leonardo da Vinci; map making of the earth and heavens was a long-standing enterprise; and geometric diagrams had been around since Euclid. Moreover, illustrations of flora and fauna, as in Hooke's Micrographia, were on a par with anything produced by graphic artists today. Nonetheless, illustrations and tables are relatively scarce into the nineteenth centuries, in part because of the expense involved in reproduction. It is not uncommon to find, for example, all the illustrations for an annual volume of a journal isolated in a slim section at the end. The graph, invented in the late eighteenth century, revealed the power of visualizations for conveying masses of data at a glance and uncovering data trends. In the nineteenth and twentieth centuries, scientists have invented many other new forms of scientific visualization, some of them requiring highly specialized decoding abilities on the part of the intended audience. Because of their utility for creating and communicating science, combined with advances in photographic reproduction and computer technology, visual representations now play a central role in the scientific article.The languages of science
English, French and Latin were the dominant languages of the scientific article in the seventeenth and eighteenth centuries. Despite the important contributions by natural philosophers throughout Europe, the fact remains that the work by investigators in countries like Italy, Spain, Denmark, Russia, and Sweden, though often published in their respective vernaculars, was usually communicated to Europe, and to the world, in one of the three major languages of science. Moreover, the two centers of scientific activity during this early period, England and France, greatly preferred publication in the vernacular over Latin, reflecting "a decisive switch from dry and bloodless scholastic erudition toward a mixed scientific/technological literature based upon the experience of the artisan, the practitioner, the traveler" (H. Floris Cohen, The Scientific Revolution: A Historiographical Inquiry, 1994). With the emergence of the German states as a center of eighteenth-century research, German became a major language of science along with English and French. In the twentieth century, as science has become a global phenomenon, scientific articles in Chinese, Japanese, and Russian also have become prominent. Nonetheless, at no other time in the long history of the scientific article has one language, what the linguist M. A. K. Halliday aptly calls "scientific English," so dominated this genre. The most-significant journals of science, whatever their nationality, now publish in English. Even the French Académie des Sciences in its Comptes rendus has turned to publishing summaries and whole articles in English. It is no accident that, when the National Research Council of Japan launched several new specialized journals in the 1920s, they chose English to reach the widest possible international audience. What's next? Now, in the 20th century, we look forward to applying electronic technology to scientific communication. Certainly the editors and scientists who started this whole process more than 300 years ago would be astounded at the scope of the worldwide scientific endeavor. But I doubt that they would have any difficulty recognizing the basically similar characteristics of the system they started. --Eugene Garfield
Current Contents (February 25, 1980)  |
 Founded in 1895, The Astrophysical Journal now publishes an electronic edition that provides articles in HTML and PDF formats. Founded in 1895, The Astrophysical Journal now publishes an electronic edition that provides articles in HTML and PDF formats. |
The scientific article is in the midst of a radical transformation spurred by advances in computer technology, in particular, word processing and graphics software, electronic mail, and the World Wide Web. This technology is changing the way in which the scientific manuscript is prepared by authors, put through peer review, produced in final form, distributed to interested readers, and perused by those readers. At the research fronts of fast-moving fields like particle physics, this shift is well on its way: scientific articles published in hardcopy periodicals are already yesterday's news. The next century, what Steven Harnad calls the "post-Gutenberg era," may very well witness the extinction of the original scientific "paper" appearing on paper. And the long-term effect of electronic preparation and publication of manuscripts may be as profound as when the scientific article evolved from scholarly letter writing and books in the seventeenth century. |
 Nature posted the results of the International Human Genome Sequencing Consortium online, free to the public. Nature posted the results of the International Human Genome Sequencing Consortium online, free to the public. |
Take, for example, the initial results of the International Human Genome Sequencing Consortium, posted online in February 2001. For publication of this instant classic, Nature magazine suspended its usual practice of allowing only subscribers access to its electronic contents, and released it to the public. We suspect this article holds the record for the longest ever published by Nature magazine (62 pages), where articles seldom exceed four or five pages. Its decoding of the 3.2 billion letters in the DNA of the human genome is the work of 20 laboratories and more than 250 scientists and computer experts around the world. The consortium's most surprising finding (though very tentative) was that the human genome has 30,000-40,000 protein-encoding genes--a far cry from the more than 100,000 previously estimated. Indeed, this number is not all that much higher than less complex forms of life like fruit flies (13,000), microscopic round worms (19,000), and mustard weeds (26,000). This long article ends by echoing the first sentence in the next to last paragraph of Watson and Crick's famous DNA article from the 1953 issue of Nature: "Finally, it is has not escaped our notice that the more we learn about the human genome, the more there is to explore" (italics indicate repetition of wording between the two articles). That sentence is followed by an apt quotation from T. S. Eliot's "Four Quartets": We shall not cease from exploration.
And the end of all our exploring
Will be to arrive where we started,
And know the place for the first time. |
ABOUT THE AUTHORJoseph E. Harmon
Joseph E. Harmon is a senior writer/editor for the Energy Technology Division of Argonne National Laboratory, a US Department of Energy laboratory operated by the University of Chicago. As an independent scholar, he has published numerous articles related to scientific communication, with emphasis on its historical development. He also collaborated with Alan G. Gross and Michael Reidy on Communicating Science: The Scientific Article from the 17th Century to the Present (Oxford University Press, 2002).
ABOUT THE AUTHORAlan G. Gross
Alan G. Gross is a professor in the Department of Rhetoric, University of Minnesota. His books include The Rhetoric of Science (2nd ed., Harvard University Press, 1996), Rhetorical Hermeneutics: Invention and Interpretation in the Age of Science (editor with William M. Keith, SUNY Press, 1996), and Rereading Aristotle's Rhetoric (editor with Arthur E. Walzer, Southern Illinois University Press, 2000). He also collaborated with Joseph E. Harmon and Michael Reidy on Communicating Science: The Scientific Article from the 17th Century to the Present (Oxford University Press, 2002).
COPYRIGHT
This seminar is based on an exhibition held in the Special Collections Research Center of the University of Chicago's Joseph Regenstein Library, May 5-August 21, 2000. Copyright 2002 the University of Chicago.


![]() Representation of two fetuses establishing a cause for their demise--a knot in the umbilical cord.
Representation of two fetuses establishing a cause for their demise--a knot in the umbilical cord.
![]() Map illustrating the past history of volcanic activity in France.
Map illustrating the past history of volcanic activity in France.
![]() Image Gallery--Early Scientific Journals
Image Gallery--Early Scientific Journals ![]()
![]()
![]()
![]()
![]()
![]()


![]() Hibiscus from Curtis's Botanical Magazine.
Hibiscus from Curtis's Botanical Magazine.
![]() A journal containing articles by Charles Darwin and Alfred Wallace.
A journal containing articles by Charles Darwin and Alfred Wallace.
![]() First edition of a lecture on relativity given by Albert Einstein in 1920.
First edition of a lecture on relativity given by Albert Einstein in 1920.![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()

![]() Hidden within a diagram of an apparatus from a 1955 article is a tiny fisherman.
Hidden within a diagram of an apparatus from a 1955 article is a tiny fisherman.
![]() Ways of organizing and presenting scientific information continue to evolve, as can be seen in the Royal Chemical Society's Visual Elements Periodic Table.
Ways of organizing and presenting scientific information continue to evolve, as can be seen in the Royal Chemical Society's Visual Elements Periodic Table.
![]() Torbern Bergman's table was an early attempt to represent chemical equations.
Torbern Bergman's table was an early attempt to represent chemical equations.![]()
![]()
![]()
![]()
![]()
![]()

![]() Founded in 1895, The Astrophysical Journal now publishes an electronic edition that provides articles in HTML and PDF formats.
Founded in 1895, The Astrophysical Journal now publishes an electronic edition that provides articles in HTML and PDF formats.
![]() Nature posted the results of the International Human Genome Sequencing Consortium online, free to the public.
Nature posted the results of the International Human Genome Sequencing Consortium online, free to the public.






